Home / Health / FDA Mandates Warning for Pfizer Birth Control
FDA Mandates Warning for Pfizer Birth Control
17 Dec, 2025
Summary
- FDA approves label change for Pfizer's Depo-Provera birth control.
- Lawsuit claims Pfizer knew about meningioma risk and failed to warn.
- International agencies in Europe, Canada, and South Africa issued warnings.
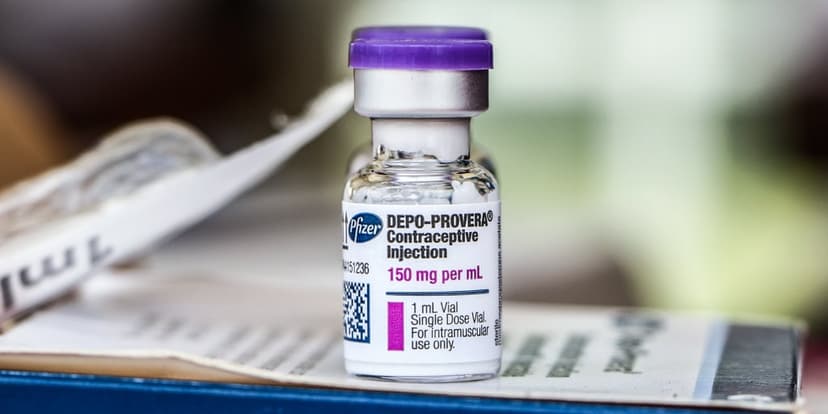
The U.S. Food and Drug Administration has approved a significant label update for Pfizer's Depo-Provera birth control injection, now explicitly warning patients about the risk of meningioma, a tumor affecting the brain's lining. This regulatory action comes amidst ongoing legal battles, with over 1,000 women suing Pfizer. They allege the pharmaceutical giant was aware of the potential tumor risk associated with the drug and deliberately failed to inform patients.
Legal filings by the plaintiffs cite studies dating back to 1983 that indicated a link between progesterone and meningiomas, suggesting Pfizer had a duty to investigate these risks sooner. Meanwhile, Pfizer has sought to dismiss the lawsuit, asserting they only became aware of the risks in 2023 and promptly applied for the FDA warning. However, the FDA initially denied a warning request for related pill formulations.
Internationally, warnings regarding meningioma risk associated with progestin-based drugs, including those containing medroxyprogesterone acetate, have already been implemented. The European Medicines Agency, Health Canada, and South Africa's drug regulatory agency have updated their respective drug labels. The U.S. judge presiding over the lawsuit has yet to issue a decision.