Home / Health / FDA Greenlights New Drugs for Superbug Gonorrhea
FDA Greenlights New Drugs for Superbug Gonorrhea
13 Dec, 2025
Summary
- Two new antibiotics, zoliflodacin and gepotidacin, were approved by the FDA.
- Gonorrhea has become increasingly resistant to existing antibiotic treatments.
- The new drugs offer hope against a growing global threat of antimicrobial resistance.

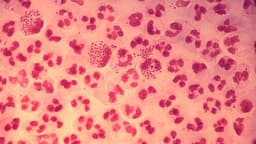
The Food and Drug Administration has given the green light to two new antibiotics, zoliflodacin and gepotidacin, offering a significant advancement in the fight against gonorrhea. This sexually transmitted infection, caused by the bacterium Neisseria gonorrhoeae, has become notoriously resistant to many existing treatments, posing a growing global health challenge. The approval of these novel drugs marks a crucial victory in combating antimicrobial resistance.
Zoliflodacin received approval following a clinical trial demonstrating its safety and efficacy as a single oral dose. Gepotidacin, previously approved for urinary tract infections, also gained expanded use for gonorrhea. Infectious disease experts have expressed optimism, hailing the new treatments as a cause for celebration in the face of escalating antibiotic resistance, which complicates medical procedures and threatens public health.