Home / Health / India's Heart Tech Shines Globally
India's Heart Tech Shines Globally
25 Nov
Summary
- Indian Myval THV series showed comparable efficacy to global leaders.
- LANDMARK trial compared Indian tech against Edwards and Medtronic.
- One-year outcomes presented at global structural heart conference.
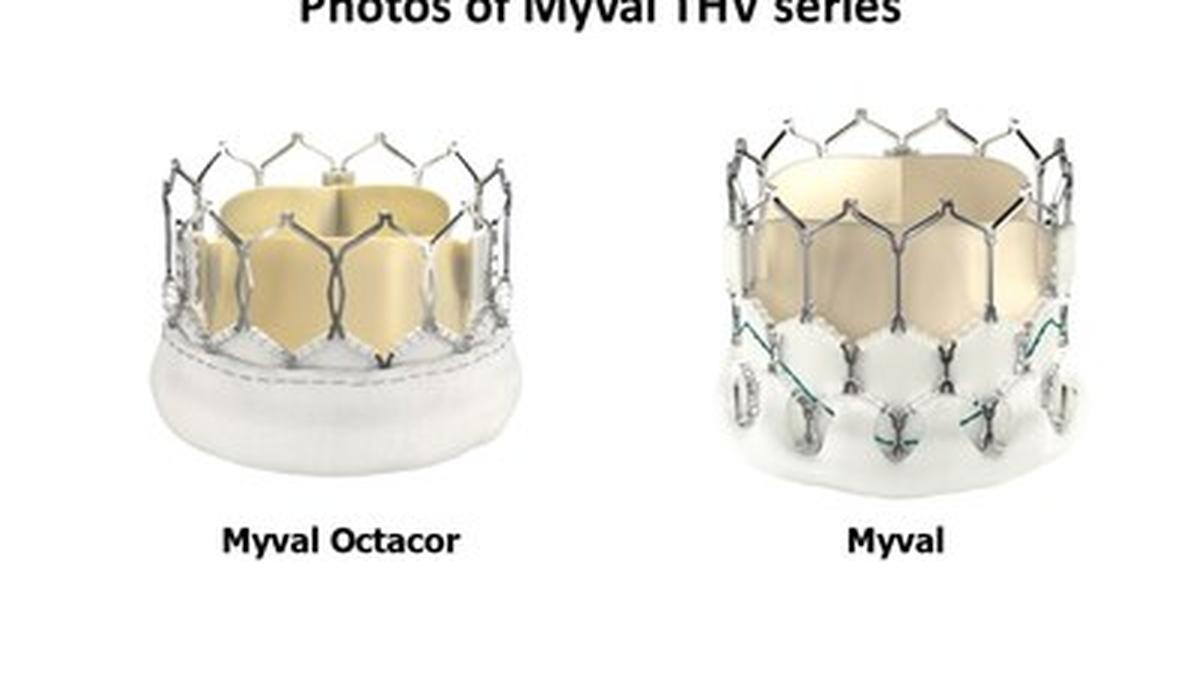
Meril Life Sciences has showcased the one-year outcomes of its pivotal LANDMARK Randomized Controlled Trial, highlighting India's growing prowess in cardiovascular innovation. Presented at PCR London Valves 2025, the trial directly compared the indigenous Myval THV series against established international transcatheter valve platforms.
The landmark study revealed that the Myval THV series demonstrated comparable clinical efficacy at one year, achieving 87% freedom from adverse events, on par with competitors. This robust performance in a large-scale, multicenter trial involving 768 patients across 16 countries positions India as a key contributor to global cardiovascular research.
These findings signal a paradigm shift in the perception of Indian medical devices, particularly in high-precision areas like structural heart therapy. The success of the Myval THV series validates India's capacity to engineer advanced cardiac solutions that meet global standards for safety, efficacy, and long-term reliability, solidifying its presence in the international MedTech arena.




