Home / Health / FDA Probes Infant RSV Drugs Amid Safety Review
FDA Probes Infant RSV Drugs Amid Safety Review
9 Dec, 2025
Summary
- FDA reviews safety of two approved infant RSV drugs.
- Drugmakers report no safety issues with Beyfortus and Enflonsia.
- Expert fears review may sow distrust in vital immunizations.
The Food and Drug Administration (FDA) has launched a safety review concerning two recently approved respiratory syncytial virus (RSV) drugs intended for infants: Beyfortus and Enflonsia. Both medications are monoclonal antibodies that provide passive immunization against RSV. Despite the ongoing review, both Sanofi, maker of Beyfortus, and Merck, maker of Enflonsia, have affirmed that no safety concerns have been identified with their respective products.
Company representatives highlighted the extensive safety data supporting their drugs, with Sanofi noting over 400,000 infants involved in clinical and real-world studies and Merck emphasizing the high standards for preventive therapies. The FDA, through a Department of Health and Human Services spokesperson, stated that it routinely evaluates all products to ensure decisions are evidence-based and in patients' best interests, with labeling updates considered if warranted.
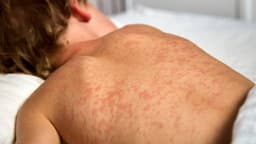
Experts like Dr. Sean O'Leary worry the FDA's review, occurring amidst other immunization policy shifts, could erode public confidence in vaccines. He predicts that decreased use of these RSV drugs would likely result in more infant hospitalizations, as RSV affects approximately two to three in every 100 infants under six months old annually, with severe cases leading to pneumonia or breathing difficulties.