Home / Health / AI Detects Fetal Abnormalities: FDA Clears Biotics AI
AI Detects Fetal Abnormalities: FDA Clears Biotics AI
19 Jan
Summary
- Biotics AI's software, using AI, detects fetal abnormalities in ultrasounds.
- The technology aims to improve prenatal care outcomes in the U.S.
- FDA clearance took nearly three years of rigorous testing and validation.
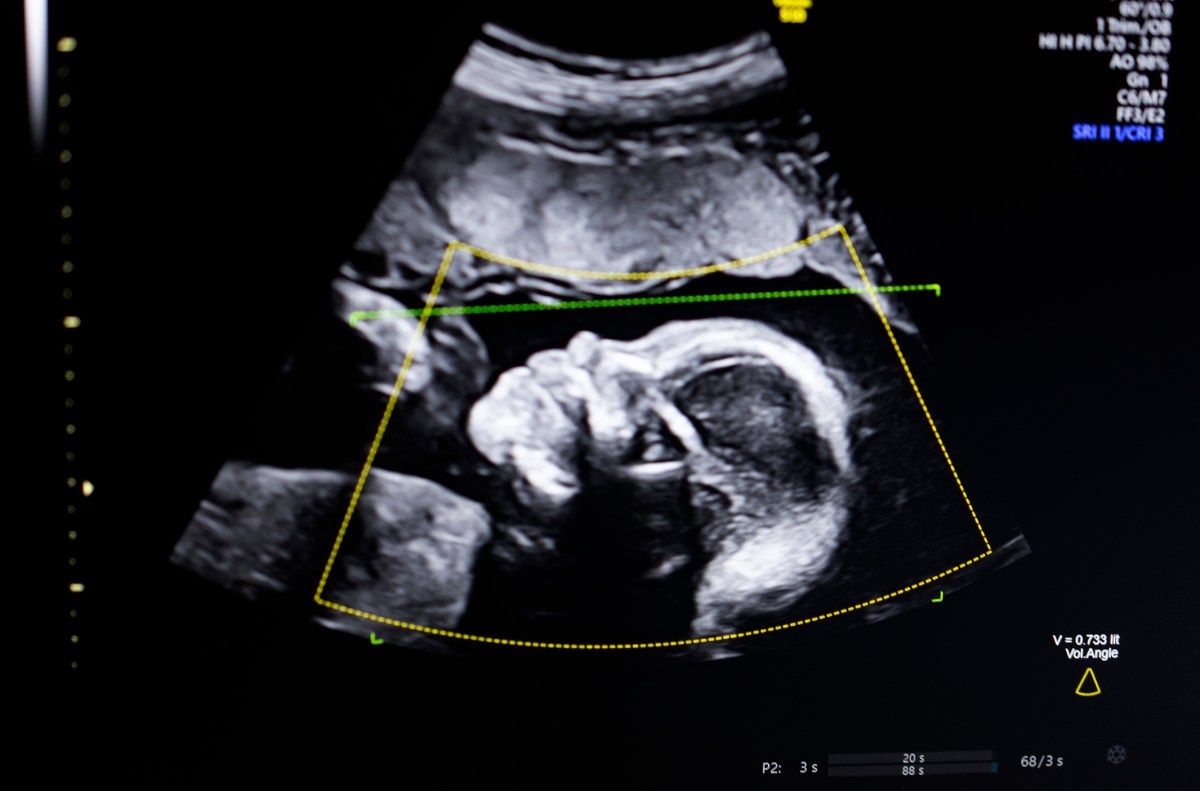
Biotics AI has achieved a significant milestone with FDA clearance for its artificial intelligence software that aids in detecting fetal abnormalities from ultrasound images. This innovative technology, envisioned by CEO Robhy Bustami and launched in 2021, employs advanced computer vision to enhance ultrasound quality assessment, ensure anatomical completeness, and automate reporting within clinical workflows.
The company's effort is driven by a mission to improve the U.S.'s prenatal care outcomes, which are notably poorer than in other high-income nations. Bustami highlighted the critical need for reliable technology, especially for demographics facing the highest risks, after training the AI on 11,000 diverse ultrasounds.
Securing FDA clearance involved nearly three years of meticulous testing and validation, emphasizing the importance of integrated engineering, product, clinical, and regulatory efforts. Biotics AI now aims to expand its reach across health systems nationwide, with future plans to enhance its offerings in fetal medicine and reproductive health.




