Home / Health / First Ever Treatment for Menkes Disease Approved
First Ever Treatment for Menkes Disease Approved
13 Jan
Summary
- Zycubo, a copper replacement therapy, is now approved.
- It's the first and only treatment available for Menkes disease.
- FDA approval brings hope to paediatric patients with the rare disorder.
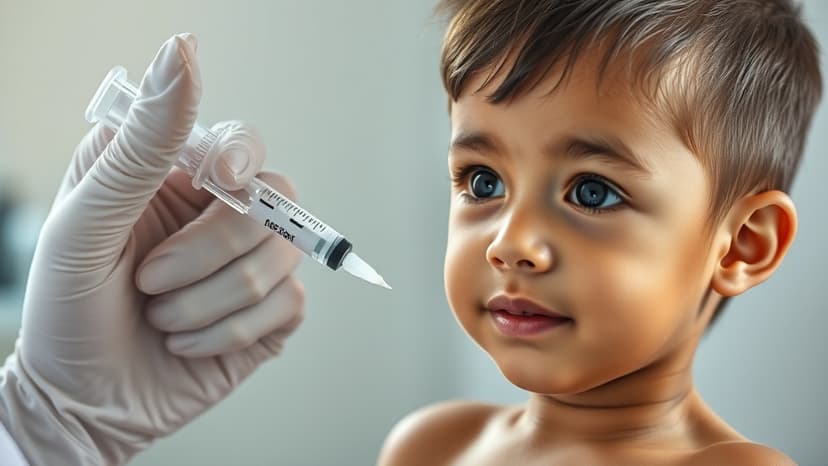
The U.S. Food and Drug Administration has granted approval for Zycubo (copper histidinate), a groundbreaking treatment for Menkes disease, developed by Sentynl Therapeutics Inc., a wholly-owned subsidiary of Zydus Lifesciences. This signifies the first and only approved therapy for this rare and fatal genetic disorder in the United States, offering a vital new option for affected children.
Menkes disease is a severe neurodegenerative disorder caused by a genetic defect that hinders copper absorption. It manifests with symptoms such as developmental delays, seizures, and significant physical abnormalities. Zycubo functions as a copper replacement therapy, administered through subcutaneous injections, addressing the core deficiency of the disease.
Sentynl Therapeutics acquired Zycubo in 2023 and successfully navigated its final development stages with the FDA. The drug has also received orphan designation from the European Medicines Agency, indicating its potential global impact in treating this debilitating condition.