Home / Health / Ozempic Knock-Offs: Are They Safe?
Ozempic Knock-Offs: Are They Safe?
18 Dec
Summary
- Compounded semaglutide lacks FDA approval for safety and effectiveness.
- The semaglutide shortage resolved in February 2025; alternatives are available.
- Risks include contamination, dosage errors, and unverified ingredients.
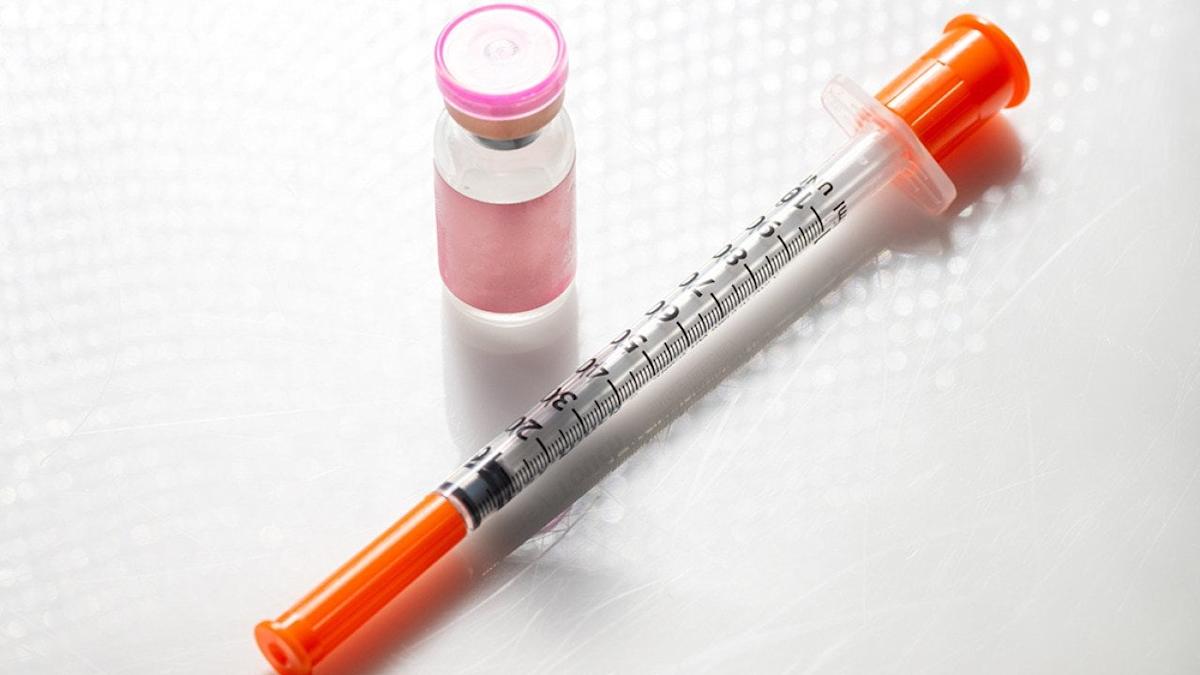
Compounded semaglutide, created by specialized pharmacies, is not subject to the same rigorous FDA testing as brand-name drugs like Ozempic and Wegovy. While these custom medications emerged during a past shortage, the FDA declared the semaglutide shortage resolved in February 2025, making FDA-approved options readily available again. The use of compounded semaglutide presents potential risks due to unverified ingredients, possible contamination, and dosage inaccuracies.
Unlike FDA-approved semaglutide injections which are pre-filled pens, compounded versions often come in vials requiring syringe measurement, increasing the chance of errors. Furthermore, compounded semaglutide is available in various forms such as sublingual drops or nasal sprays, for which clinical studies on safety and effectiveness are lacking. Concerns also exist regarding the use of semaglutide salts and ingredients sourced from unauthorized or nonpharmaceutical facilities.