Home / Health / WHO Greenlights Pneubevax 14 for Global Use
WHO Greenlights Pneubevax 14 for Global Use
21 Nov
Summary
- Biological E.'s Pneubevax 14 vaccine received WHO pre-qualification.
- The vaccine protects against 14 different pneumococcal serotypes.
- It is administered to infants from six weeks of age.
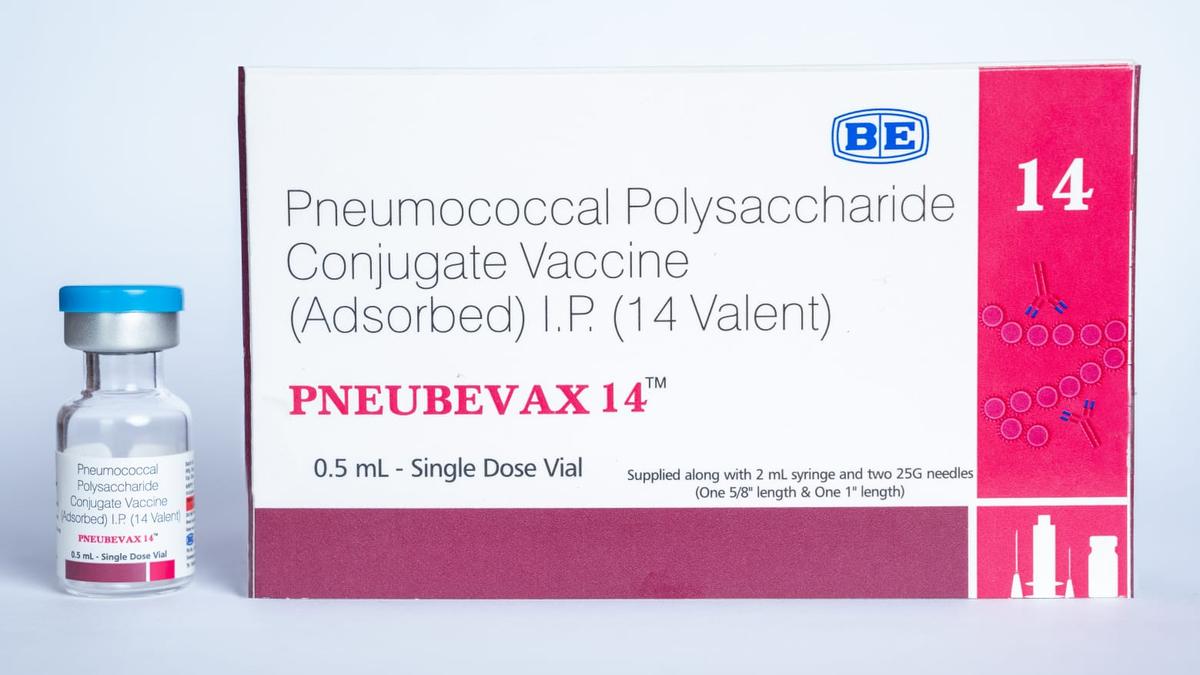
Biological E.'s innovative 14-valent pneumococcal conjugate vaccine, Pneubevax 14, has secured crucial World Health Organization (WHO) pre-qualification status. This achievement significantly broadens its potential reach through global immunization programs, aiming to improve access to high-quality vaccines worldwide and bolster supply chains for vulnerable populations, especially children. The company views this as a vital step in its commitment to public health.
Pneubevax 14 is the eleventh vaccine from Biological E. to earn WHO pre-qualification. It is designed to combat invasive pneumococcal diseases by targeting 14 distinct serotypes of Streptococcus pneumoniae. Notably, it includes protection against serotypes 22F and 33F, which are not covered by certain other available vaccines, offering a more comprehensive shield.
The vaccine is suitable for infants as young as six weeks old and is intended for use within primary vaccination schedules. Clinical trials have demonstrated Pneubevax 14's favorable safety profile and its capacity to elicit strong immune responses against all 14 targeted strains, making it a vital tool in the fight against serious infections like pneumonia, meningitis, and sepsis.