Home / Health / Leukaemia Cure: NHS Approves Groundbreaking Cell Therapy
Leukaemia Cure: NHS Approves Groundbreaking Cell Therapy
25 Nov
Summary
- A new one-time cell therapy, obe-cel, has been approved by NHS.
- The treatment genetically modifies cells to target leukaemia effectively.
- Clinical trials show high remission rates, with 77% achieving remission.
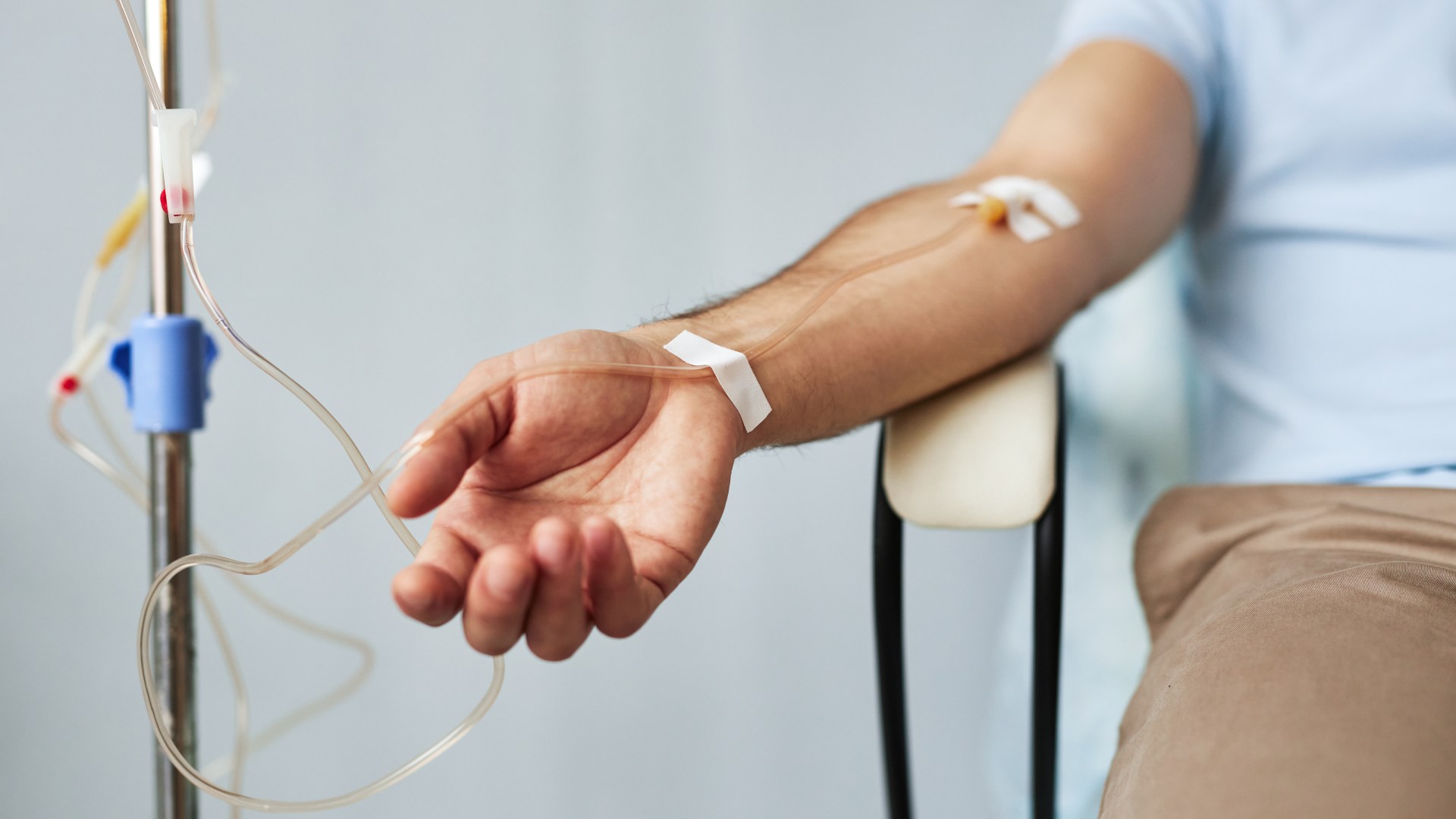
A revolutionary treatment for leukaemia, known as obe-cel or Aucatzyl, has been granted NHS approval in England, offering new hope to over 150 patients annually. Developed by Autolus, a spin-out from University College London, this therapy involves a single course of intravenous doses designed to genetically modify a patient's cells.
The modified cells then enable the immune system to recognize and attack cancerous leukaemia cells. This innovative approach has shown remarkable efficacy in clinical trials, particularly for patients whose cancer has relapsed or resisted initial treatments. High remission rates and sustained remission have been observed, suggesting a potential functional cure.
Evidence from a trial involving 94 individuals revealed that 77% achieved remission, with a significant portion remaining cancer-free for over three years. This approval by the National Institute for Health and Care Excellence represents a substantial advancement in the fight against leukaemia, with similar treatments also available for younger patients.