Home / Health / Marea's MAR002 Shows Strong IGF-1 Reduction in Trials
Marea's MAR002 Shows Strong IGF-1 Reduction in Trials
14 Jan
Summary
- MAR002 demonstrated significant, dose-dependent IGF-1 reduction.
- Trial data shows MAR002 superior to current treatments.
- Marea Therapeutics advances MAR001 and siRNA programs in 2026.
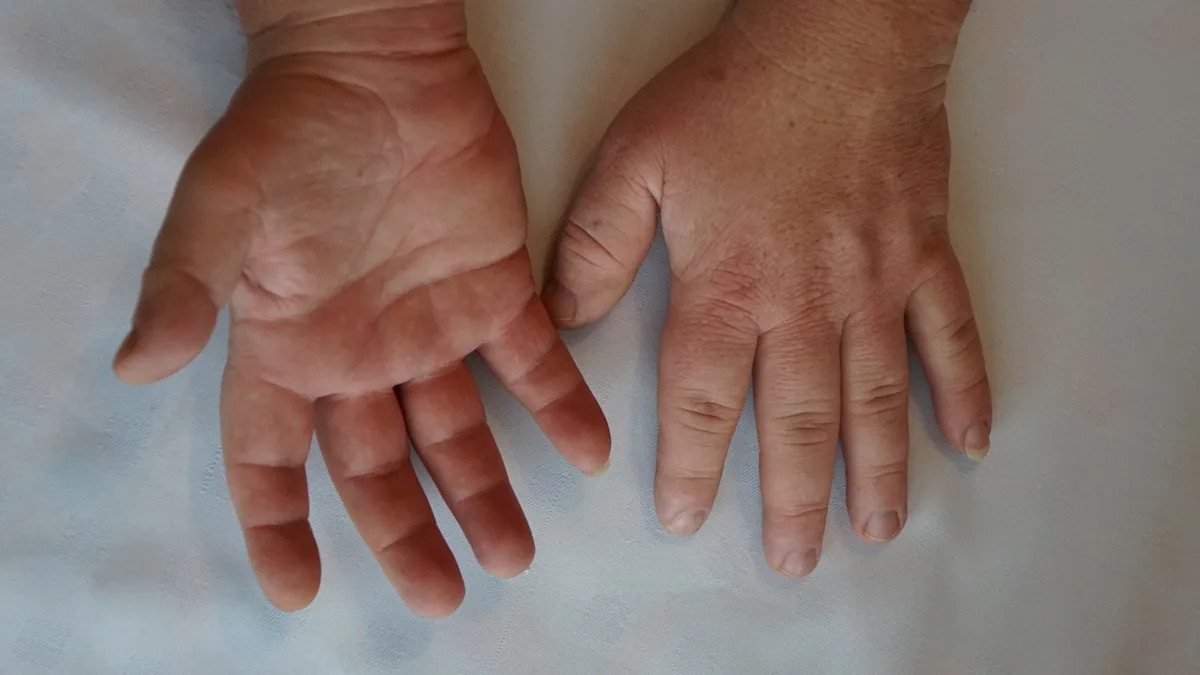
Marea Therapeutics has reported favorable top-line results from its initial Phase I study of MAR002, a novel antibody targeting the growth hormone receptor for acromegaly treatment. The trial, conducted in healthy volunteers, demonstrated a good safety profile and significant, dose-dependent reductions in IGF-1 levels.
Notably, MAR002 achieved over 45% IGF-1 suppression lasting up to 43 days at the highest dose, outperforming existing treatments in both magnitude and duration. This validates MAR002's mechanism and suggests suitability for bi-weekly or monthly dosing.
Looking ahead to 2026, Marea is advancing MAR001, a monoclonal antibody for cardiometabolic disorders, with Phase IIb study enrollment concluding by January 2026 and data expected mid-year. The company is also progressing a preclinical siRNA program targeting ANGPTL4, aiming for candidate nomination within the year.