Home / Health / HPV Vaccine Schedule Cut: Independent Review Launched
HPV Vaccine Schedule Cut: Independent Review Launched
8 Jan
Summary
- Independent group reviews single-dose HPV vaccine given to children.
- CDC's HPV vaccine change bypassed traditional expert review.
- Merck's Gardasil vaccine sales reached $2.4 billion in 2024.

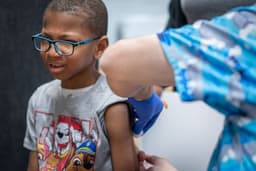
An independent vaccine advisory group has initiated a scientific evidence review concerning the human papillomavirus (HPV) vaccine. This action follows a recent recommendation by U.S. health officials to administer the vaccine as a single dose, a departure from the U.S. Food and Drug Administration's approval for multiple doses. The U.S. Centers for Disease Control and Prevention's revised schedule suggests children receive one dose at age 11, deviating from the previously recommended two or three doses.
This significant revision to the childhood vaccine schedule notably bypassed the traditional review process, which typically involves extensive evaluation by outside experts. Instead, the agency based its decision on a review by senior Health and Human Services staff, reportedly influenced by President Donald Trump's push to reduce childhood vaccinations. The Vaccine Integrity Project, established to provide independent reviews, aims to ensure policymakers and the public have accurate data interpretations.
The Vaccine Integrity Project is examining evidence that a single dose may suffice, a view supported by the World Health Organization. However, critical questions remain regarding the single dose's appropriateness for older adolescents, immunocompromised individuals, and its long-term efficacy. Merck's Gardasil, the sole U.S.-licensed HPV vaccine, is approved based on two doses, and the company has stated insufficient data exists for single-dose licensing. Merck recorded $2.4 billion in U.S. Gardasil sales in 2024.