Home / Health / Hep B Vaccine Policy Under Fire: Experts Rebut Claims
Hep B Vaccine Policy Under Fire: Experts Rebut Claims
2 Dec
Summary
- Newborn hepatitis B vaccine policy cut infections by over 95%.
- Experts review found no safety data to delay infant vaccination.
- Delaying vaccine risks increased infant infections from parents.
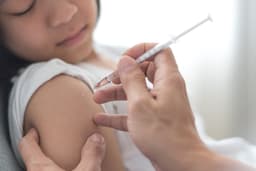
An independent review of hundreds of studies by vaccine experts has concluded that the long-standing U.S. policy of administering the hepatitis B vaccine to newborns is highly effective, cutting infections by over 95%. The review found no safety or effectiveness data to support delaying this vital vaccination for infants.
The policy, established in 1991, has recently faced scrutiny, with calls from some health officials to postpone the birth dose until as late as age 12. However, experts assert that delaying the vaccine significantly increases the risk of newborns contracting hepatitis B from infected parents or caregivers.
Infants infected in their first year have a high chance of developing chronic liver disease. The review emphasizes that keeping the current policy is strongly supported by evidence, safeguarding public health against this serious liver infection.