Home / Health / Delgocitinib Cream Approved for NHS Use, Offering Relief to 62,000 Eczema Patients
Delgocitinib Cream Approved for NHS Use, Offering Relief to 62,000 Eczema Patients
13 Nov
Summary
- New eczema cream delgocitinib approved for NHS use
- Cream helps treat chronic hand eczema, a debilitating condition
- Potential to save NHS millions of pounds
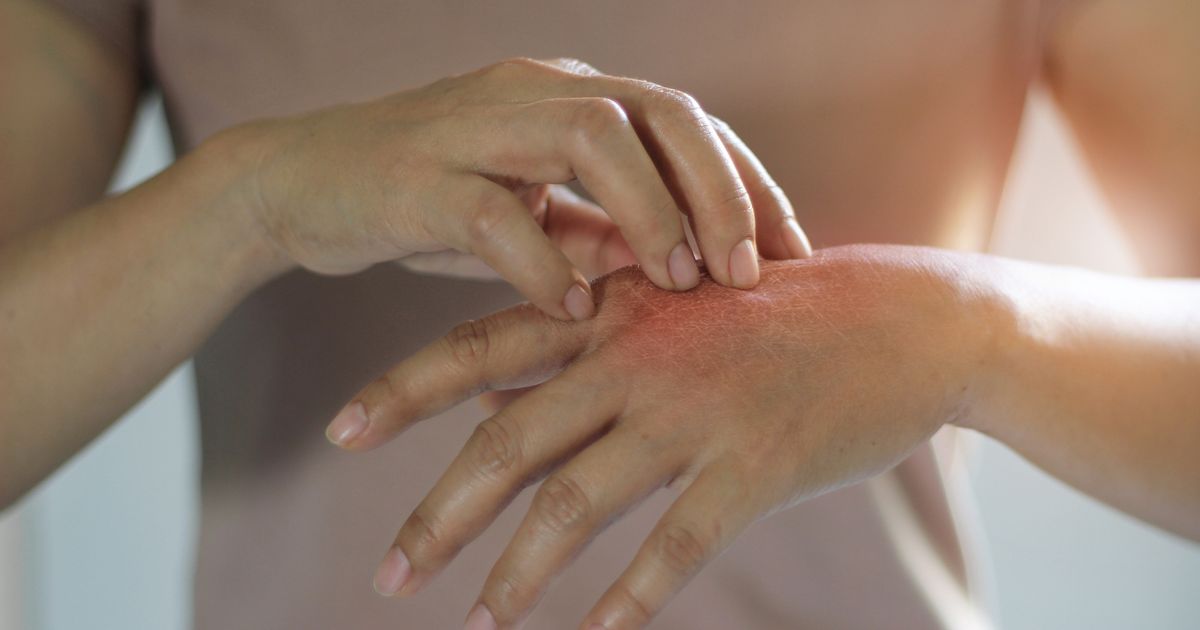
On November 13th, 2025, a promising update was shared regarding a new drug approved for the treatment of eczema, a debilitating skin condition. The National Institute for Health and Care Excellence (Nice) has green-lighted delgocitinib, also known as Anzupgo, a cream produced by Leo Pharma, for NHS use.
This new cream could be a game-changer for the over 62,000 people in England suffering from moderate to severe chronic hand eczema. This condition causes the hands to become dry, sore, cracked, and itchy, making everyday tasks challenging. The cream, which is applied twice daily to the affected areas, has the potential to save the NHS millions of pounds.
For those who work outside or need to wash their hands regularly, such as healthcare workers, this new treatment could be life-changing. Unlike current options like ultraviolet light therapy or retinoid medication, which can have unpleasant side effects, delgocitinib offers a more convenient and effective solution that can be used at home.
According to BBC health expert Dr. Oscar Duke, this new cream could be particularly helpful for those whose skin has become very thickened, as traditional steroid creams often fail to provide relief. With delgocitinib now available on the NHS within the next 90 days, thousands of eczema patients in England can look forward to a brighter future.




