Home / Health / FDA Expands Libido Pill Access Post-Menopause
FDA Expands Libido Pill Access Post-Menopause
16 Dec
Summary
- FDA broadens Addyi's use to women over 65 post-menopause.
- Addyi carries a warning for dangerous interactions with alcohol.
- Low libido drug development surged after Viagra's success.
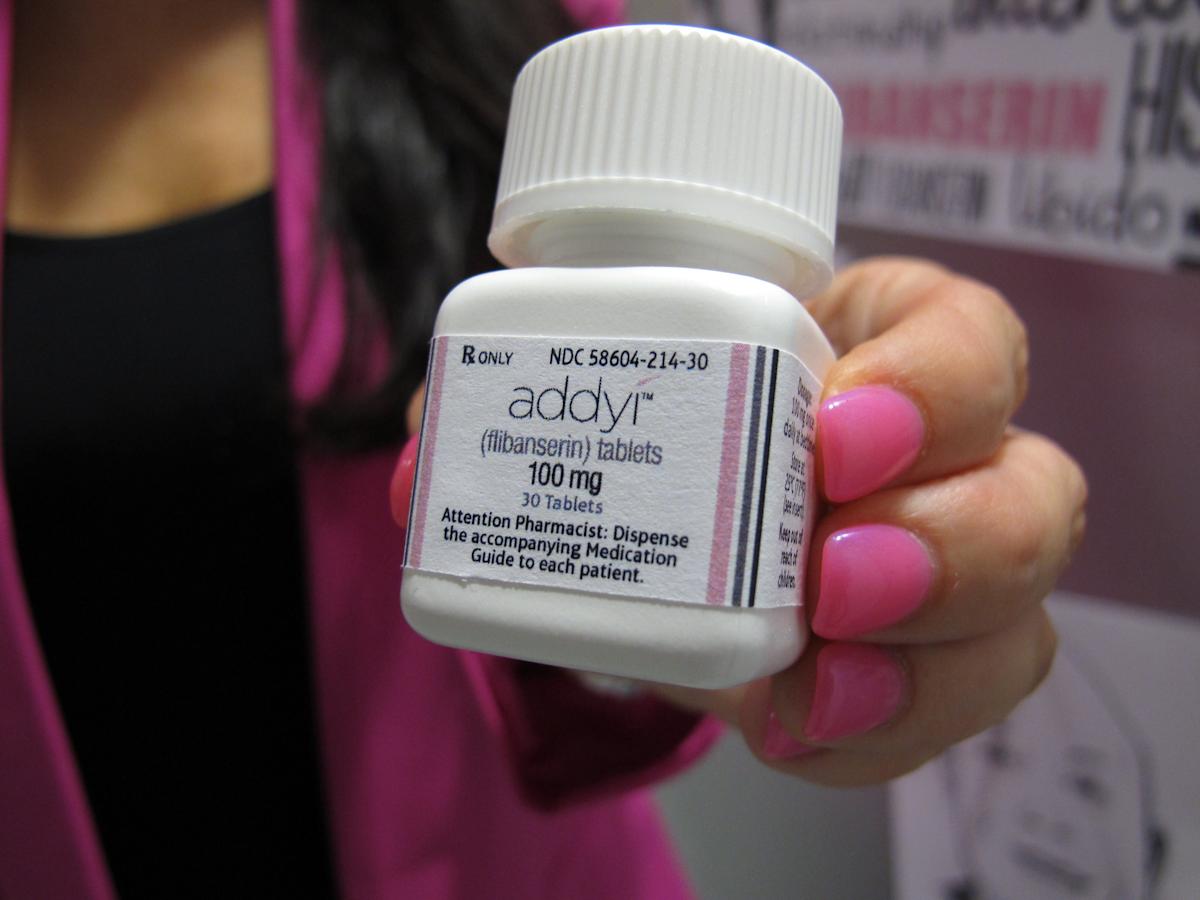
U.S. health officials have expanded the approval of Addyi, a medication designed to boost libido in women. The Food and Drug Administration announced that the once-daily pill can now be prescribed to women over the age of 65 who have completed menopause. This decision broadens the accessibility of the drug, which was first approved a decade ago for premenopausal women experiencing emotional distress due to low sex drive.
Addyi, marketed by Sprout Pharmaceuticals, was initially anticipated to be a major success in women's health. However, its limited sales can be attributed to notable side effects like dizziness and nausea. Critically, the drug carries a serious safety warning regarding the consumption of alcohol, which can lead to dangerously low blood pressure and fainting. This cautionary note underscores the complex safety profile of the medication.
The medical condition characterized by a significantly low sexual appetite, known as hypoactive sexual desire disorder, has been recognized since the 1990s. Drugmakers began investing heavily in female sexual dysfunction therapies following the immense success of Viagra for men. Despite these efforts, diagnosing low libido in women can be challenging due to various factors, including hormonal changes after menopause and other medical or psychological conditions.



