Home / Health / FDA Approves Alfapump for Liver Cirrhosis Relief
FDA Approves Alfapump for Liver Cirrhosis Relief
25 Nov, 2025
Summary
- First alfapump implantation occurred at Mount Sinai Hospital in the U.S.
- The device automatically removes ascites fluid for natural elimination.
- It aims to significantly improve quality of life for patients.

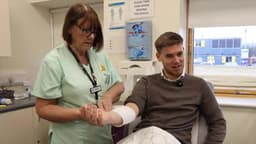
A significant advancement in medical technology, the alfapump, has received FDA approval for the treatment of ascites related to liver cirrhosis in the United States. This innovative device was first implanted at Mount Sinai Hospital, marking a new era for patients suffering from this condition.
The alfapump offers a revolutionary approach by automatically removing ascitic fluid from the abdomen. This fluid is then directed to the bladder for natural elimination through urination, effectively ending the need for repeated and invasive drainage procedures. Patients can now anticipate a life free from the burden of frequent interventions.
Dr. Rahul Patel highlighted the alfapump as a transformative technology, emphasizing its potential to enhance patient quality of life and decrease hospitalizations. This breakthrough offers a less invasive and more continuous management of ascites, providing much-needed relief and hope for those affected by this challenging medical condition.