Home / Health / EU Rejects Lilly's Heart Failure Heartbreak
EU Rejects Lilly's Heart Failure Heartbreak
30 Jan
Summary
- European regulators did not back Mounjaro for heart failure in obese adults.
- Uncertainty remains on whether weight loss drove trial's heart benefits.
- The drug's heart failure data will still be added to product information.
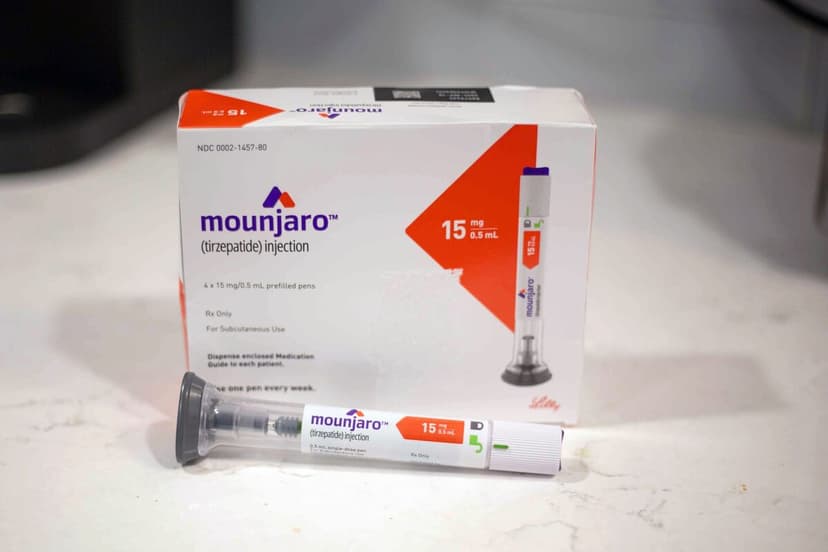
Eli Lilly & Co. has faced a setback as the European Union's medicines regulator declined to endorse its weight-loss drug, Mounjaro, for treating a specific form of heart failure in adults with obesity. The European Medicines Agency's drug advisory panel on Friday stated it remains uncertain if the trial's observed reduction in heart-failure hospitalizations was due to a mechanism separate from weight loss.
This decision impacts Lilly's strategy to expand the patient pool for its highly successful medication, known as Zepbound in the US. The pharmaceutical industry is actively seeking to broaden insurance coverage for weight-loss drugs by demonstrating their efficacy beyond weight reduction, highlighting improvements in various health components.
Although a distinct indication was not recommended, the EMA agreed to incorporate the study's relevant findings into Mounjaro's product information. The trial focused on patients with heart failure with a preserved ejection fraction (HFpEF), a condition strongly associated with obesity affecting millions globally and carrying a significant mortality rate.