Home / Health / I Got Dengue Fever to Test a New Vaccine
I Got Dengue Fever to Test a New Vaccine
18 Dec
Summary
- The author volunteered for a dengue virus trial and was injected with a modified virus.
- Ethical considerations involve risk-benefit analysis for participants and society.
- Participants can receive up to $3,425, sparking debate on compensation ethics.
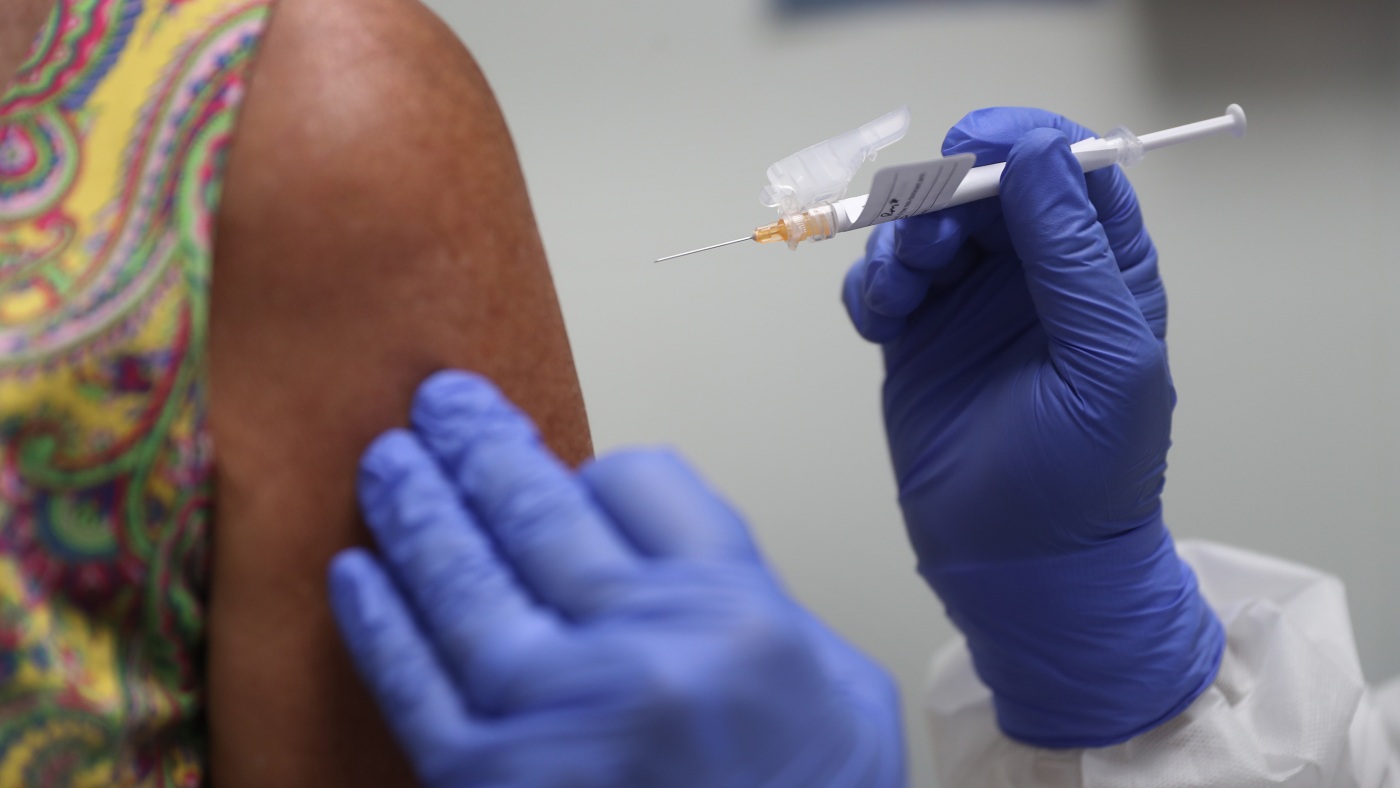
A journalist participated in a Phase 2 clinical trial at Johns Hopkins, receiving an experimental dengue virus injection to test a potential vaccine. This involved a rigorous informed consent process and enduring flu-like symptoms, along with a lifelong increased risk of severe dengue.
The trial, which involved injecting a genetically modified dengue virus, aimed to evaluate an experimental drug's efficacy. The process included multiple infusions, blood draws, and a period of illness, raising ethical questions about participant risk and societal benefit.
Compensation for participating in such trials, up to $3,425 in this case, is a subject of ethical debate. While some argue for higher pay based on time and effort, others express concern that financial incentives might unduly influence vulnerable individuals.