Home / Health / Knock-off Weight Drugs: Deadly Risks Exposed
Knock-off Weight Drugs: Deadly Risks Exposed
30 Jan
Summary
- Compounded semaglutide lacks FDA approval and poses severe health risks.
- Millions of Americans face dangers from unregulated weight loss concoctions.
- FDA reports link compounded GLP-1s to severe injuries and potential deaths.

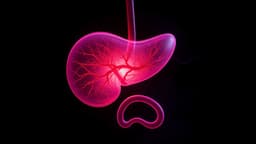
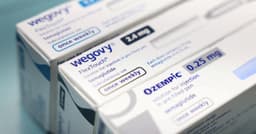
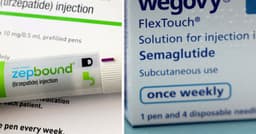
Compounded semaglutide, an unregulated version of popular weight loss drugs, is under intense scrutiny due to potential severe health risks. These non-FDA-approved concoctions, often mixed with other additives by compounding pharmacies, have surged in popularity as a lower-cost alternative to brand-name GLP-1 medications like Ozempic and Wegovy. Millions of Americans are reportedly using these drugs, which began to gain traction after shortages of the approved versions emerged in 2022.
Experts, including the FDA and the CEO of Novo Nordisk, have cautioned against their use, highlighting risks such as ingredient sourcing variability, potential dosing errors, and compromised sterility. Reports have surfaced linking compounded semaglutide to adverse events, including pancreatitis and gallbladder injury, with the FDA's MedWatch database logging several deaths potentially associated with these substances since 2023. These medications are powerful, and formulation and oversight are critical for patient safety.