Home / Health / China Approves First Homegrown Subunit Flu Vaccine
China Approves First Homegrown Subunit Flu Vaccine
2 Feb
Summary
- China's first homegrown subunit flu vaccine is now approved for use.
- The new vaccine is available for individuals aged six months and above.
- This subunit vaccine offers a purer composition and higher safety profile.
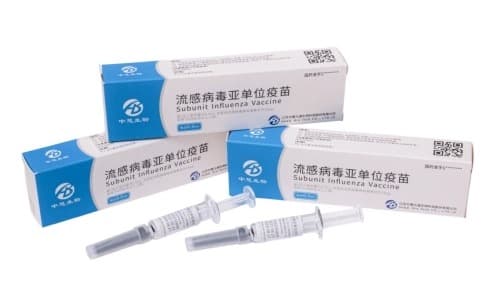
China has authorized its first homegrown subunit influenza vaccine, a significant development for the nation's public health initiatives. Developed by Ab&B Biotech, this trivalent vaccine has received market approval from the National Medical Products Administration.
The newly approved vaccine is now accessible for individuals aged six months and above, making it eligible for broad public administration. This represents a crucial step in enhancing flu prevention strategies across the country.
Subunit vaccines, like the one recently approved, utilize a more refined purification process. This results in a purer composition and a higher safety profile, according to health experts. The availability of this advanced vaccine offers more options for influenza prevention and control, both domestically and on a global scale.



