Home / Health / CDC Alters Hepatitis B Vaccine Rules for Infants
CDC Alters Hepatitis B Vaccine Rules for Infants
17 Dec
Summary
- New CDC guideline delays hepatitis B vaccine for newborns.
- Parents may opt-out of birth dose for infants of Hep B-negative mothers.
- Experts fear policy change could increase infant exposure to the virus.
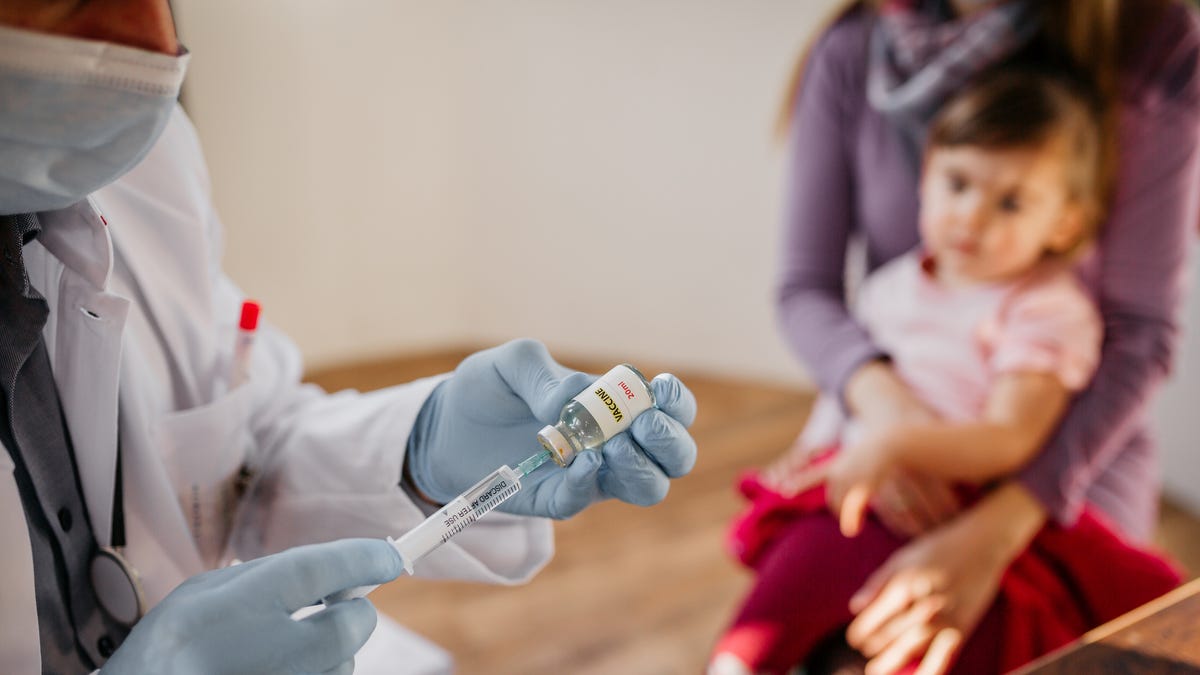
A significant policy change regarding hepatitis B vaccination in newborns was approved by the Centers for Disease Control and Prevention on December 16th. Following a recommendation from a vaccine advisory panel, the CDC has ended its long-standing guideline for universal hepatitis B vaccination at birth for all infants.
The updated recommendation allows parents, in consultation with healthcare providers, to decide if their newborn should receive the hepatitis B vaccine, particularly if the mother tests negative for the virus. Should parents choose to delay the initial dose, the CDC now suggests administering the first vaccine at least two months after birth.
This departure from the previous 1991 recommendation has raised concerns among health experts. They warn that this move toward individual-based decision-making might lead to increased exposure to the hepatitis B virus among infants and could see more families opting out of vaccination altogether.